Healthwinnow
Minoxidil/米诺地尔
Minoxidil/米诺地尔
无法加载取货服务可用情况

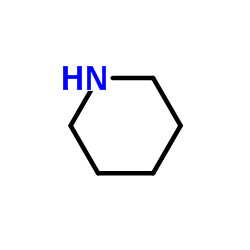
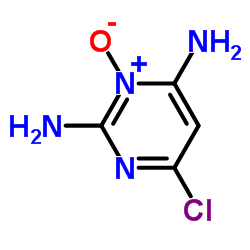
Minoxidil structure
|
Common Name | Minoxidil | ||
|---|---|---|---|---|
| CAS Number | 38304-91-5 | Molecular Weight | 209.248 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 351.7±45.0 °C at 760 mmHg | |
| Molecular Formula | C9H15N5O | Melting Point | 272-274 °C (dec.)(lit.) | |
| MSDS | ChineseUSA | Flash Point | 166.5±28.7 °C | |
| Symbol |
 GHS07 |
Signal Word | Warning | |
Use of MinoxidilMinoxidil(U 10858) is an antihypertensive vasodilator medication. Target: potassium channel Minoxidil, a potent antihypertensive agent, induces generalized hypertrichosis when administered systemically, or localized hair regrowth when applied topically to sites of severe alopecia areata. The pharmacologic mechanisms by which minoxidil stimulates hair growth are unknown. This study was designed to examine whether minoxidil has direct effects on neonatal murine epidermal cells in culture. In the presence of minoxidil, cultures showed a marked dose-dependent second peak of DNA synthesis 8-10 days after culture initiation. In addition, two morphologically distinct cell types appeared. Indirect immunofluorescence staining with keratin-specific antibody revealed cytoplasmic keratin fibers, suggesting the epidermal origin of these cells. Our experiments demonstrate that minoxidil can affect epidermal cells in culture by altering their growth pattern and phenotypic appearance [1] . Finite doses of minoxidil (2%, w/v) in formulations containing varying amounts of ethanol, propylene glycol (PG), and water (60:20:20, 80:20:0, and 0:80:20 by volume, respectively) were used. Minoxidil in SC (by tape stripping), appendages (by cyanoacrylate casting), and receptor fluid was determined by liquid scintillation counting. At early times (30 min, 2 h), ethanol-containing formulations (60:20:20 and 80:20:0) caused significantly greater minoxidil retention in SC and appendages, compared to the formulation lacking ethanol (0:80:20). A significant increase in minoxidil receptor penetration occurred with the PG-rich 0:80:20 formulation after 12 h [2]. |
| Name | minoxidil |
|---|---|
| Synonym | More Synonyms |
| Description | Minoxidil(U 10858) is an antihypertensive vasodilator medication. Target: potassium channel Minoxidil, a potent antihypertensive agent, induces generalized hypertrichosis when administered systemically, or localized hair regrowth when applied topically to sites of severe alopecia areata. The pharmacologic mechanisms by which minoxidil stimulates hair growth are unknown. This study was designed to examine whether minoxidil has direct effects on neonatal murine epidermal cells in culture. In the presence of minoxidil, cultures showed a marked dose-dependent second peak of DNA synthesis 8-10 days after culture initiation. In addition, two morphologically distinct cell types appeared. Indirect immunofluorescence staining with keratin-specific antibody revealed cytoplasmic keratin fibers, suggesting the epidermal origin of these cells. Our experiments demonstrate that minoxidil can affect epidermal cells in culture by altering their growth pattern and phenotypic appearance [1] . Finite doses of minoxidil (2%, w/v) in formulations containing varying amounts of ethanol, propylene glycol (PG), and water (60:20:20, 80:20:0, and 0:80:20 by volume, respectively) were used. Minoxidil in SC (by tape stripping), appendages (by cyanoacrylate casting), and receptor fluid was determined by liquid scintillation counting. At early times (30 min, 2 h), ethanol-containing formulations (60:20:20 and 80:20:0) caused significantly greater minoxidil retention in SC and appendages, compared to the formulation lacking ethanol (0:80:20). A significant increase in minoxidil receptor penetration occurred with the PG-rich 0:80:20 formulation after 12 h [2]. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Related Catalog | |||||||||||||
| Solvent |
In Vitro:
DMSO : 4.6 mg/mL (21.98 mM; Need ultrasonic and warming) |
||||||||||||
| Solubility |
|
||||||||||||
| Storage |
|
||||||||||||
| Shipping | Room temperature in continental US; may vary elsewhere | ||||||||||||
| SMILES | NC1=NC(N2CCCCC2)=CC(N)=[N+]1[O-] | ||||||||||||
| References | |||||||||||||
| Related Molecules | Nigericin sodium salt | Senicapoc | E-4031 | 4-AMINOPYRIDINE | Ginsenoside Rg3 | TRAM-34 | Dofetilide | PAP-1 | Flufenamic Acid | Flupirtine maleate | NS-1619 | Endoxifen (Z-isomer hydrochloride) | NS309 | Quinine | Terfenadine |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 351.7±45.0 °C at 760 mmHg |
| Melting Point | 272-274 °C (dec.)(lit.) |
| Molecular Formula | C9H15N5O |
| Molecular Weight | 209.248 |
| Flash Point | 166.5±28.7 °C |
| Exact Mass | 209.127655 |
| PSA | 91.16000 |
| LogP | -1.49 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.724 |
| Storage condition | Store at RT |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |
 GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22;R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | UV8200000 |
| HS Code | 2933990090 |
| Precursor 3 |
|
|---|---|
| DownStream 0 |
|
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Phase II metabolism in human skin: skin explants show full coverage for glucuronidation, sulfation, N-acetylation, catechol methylation, and glutathione conjugation.
Drug Metab. Dispos. 43(1) , 126-39, (2014) Although skin is the largest organ of the human body, cutaneous drug metabolism is often overlooked, and existing experimental models are insufficiently validated. This proof-of-concept study investig... |
|
|
Intestinal permeability study of minoxidil: assessment of minoxidil as a high permeability reference drug for biopharmaceutics classification.
Mol. Pharm. 12(1) , 204-11, (2015) The purpose of this study was to evaluate minoxidil as a high permeability reference drug for Biopharmaceutics Classification System (BCS). The permeability of minoxidil was determined in in situ inte... |
|
|
Fabrication of an electrochemical sensor based on computationally designed molecularly imprinted polymer for the determination of mesalamine in real samples.
Mater. Sci. Eng. C. Mater. Biol. Appl. 55 , 209-17, (2015) A novel electrochemical sensor based on mesalamine molecularly imprinted polymer (MIP) film on a glassy carbon electrode was fabricated. Density functional theory (DFT) in gas and solution phases was ... |
| Regaine |
| EINECS 253-874-2 |
| 2,4-diamino-6-piperidino-pyrimidine-3-oxide |
| 2,6-Diamino-4-piperidinopyrimidine 1-Oxide |
| Rogaine |
| 4-Pyrimidinamine, 2,3-dihydro-3-hydroxy-2-imino-6-(1-piperidinyl)-, (2E)- |
| MFCD00063409 |
| Theroxidil |
| 3-hydroxy-2-imino-6-piperidin-1-ylpyrimidin-4-amine |
| Tricoxidil |
| 6-(1-Piperidinyl)-2,4-pyrimidinediamine 3-oxide 6-(1-Piperidinyl)pyrimidine-2,4-diamine 3-oxide |
| Lonolox |
| (2E)-6-Amino-2-imino-4-(1-piperidinyl)-1(2H)-pyrimidinol |
| 6-(1-Piperidinyl)-2,4-pyrimidinediamine 3-oxide (6-(1-Piperidinyl)pyrimidine-2,4-diamine 3-oxide |
| 2,6-Diamino-4-(piperidin-1-yl)pyrimidine 1-oxide |
| Alostil |
| Loniten |
| Minoximen |

-
Shipping
Share the details of your shipping policy.
-
Returns
Share the details of your return policy.
Image with text
Pair text with an image
Pair text with an image to focus on your chosen product, collection, or artist. Add details on availability, style, or even provide a review.
Image with text
Pair text with an image to provide extra information about your brand or collections.